Calorimeter Experiment A Level . The temperature data is then plotted, and extrapolation used to determine the temperature change on reaction of the substances. In an enthalpy change practical, the temperature change needs to be accurately measured. How much the temperature of the water changes is based on how much energy is. Calorimetry is used to physically measure changes in enthalpy. Using a simple calorimeter and a bomb. a level chemistry aqa 1.4.2. the heat flows into the container of water held above the reaction. this page describes experimental methods for determining enthalpy changes of chemical reactions e.g. in this a level and ib chemistry video, hazel describes how you carry out an. the essence of calorimetry in chemistry lies in its ability to link heat changes to molar quantities. in the calorimetric experiment, substances are mixed in an insulated container, known as a calorimeter, and the temperature of the container is measured over a period of time.
from ar.inspiredpencil.com
How much the temperature of the water changes is based on how much energy is. a level chemistry aqa 1.4.2. in this a level and ib chemistry video, hazel describes how you carry out an. the heat flows into the container of water held above the reaction. Using a simple calorimeter and a bomb. Calorimetry is used to physically measure changes in enthalpy. In an enthalpy change practical, the temperature change needs to be accurately measured. in the calorimetric experiment, substances are mixed in an insulated container, known as a calorimeter, and the temperature of the container is measured over a period of time. the essence of calorimetry in chemistry lies in its ability to link heat changes to molar quantities. this page describes experimental methods for determining enthalpy changes of chemical reactions e.g.
Heat Of Combustion Lab
Calorimeter Experiment A Level the heat flows into the container of water held above the reaction. Calorimetry is used to physically measure changes in enthalpy. a level chemistry aqa 1.4.2. How much the temperature of the water changes is based on how much energy is. in the calorimetric experiment, substances are mixed in an insulated container, known as a calorimeter, and the temperature of the container is measured over a period of time. the essence of calorimetry in chemistry lies in its ability to link heat changes to molar quantities. The temperature data is then plotted, and extrapolation used to determine the temperature change on reaction of the substances. the heat flows into the container of water held above the reaction. Using a simple calorimeter and a bomb. In an enthalpy change practical, the temperature change needs to be accurately measured. this page describes experimental methods for determining enthalpy changes of chemical reactions e.g. in this a level and ib chemistry video, hazel describes how you carry out an.
From www.linstitute.net
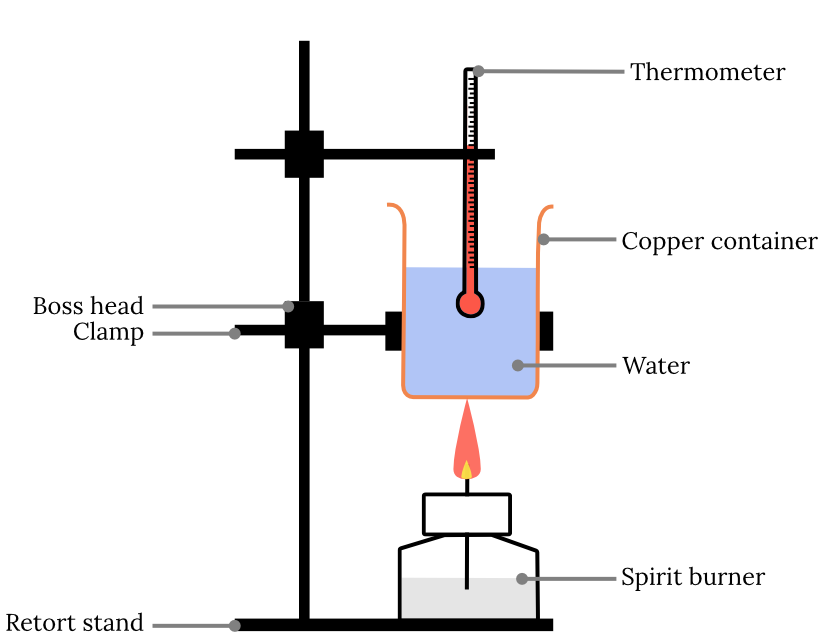
Edexcel A Level Chemistry复习笔记1.8.3 Calorimetry翰林国际教育 Calorimeter Experiment A Level In an enthalpy change practical, the temperature change needs to be accurately measured. The temperature data is then plotted, and extrapolation used to determine the temperature change on reaction of the substances. How much the temperature of the water changes is based on how much energy is. Calorimetry is used to physically measure changes in enthalpy. Using a simple calorimeter. Calorimeter Experiment A Level.
From answermagicmatney.z21.web.core.windows.net
Coffee Cup Calorimetry Experiment Calorimeter Experiment A Level the heat flows into the container of water held above the reaction. a level chemistry aqa 1.4.2. this page describes experimental methods for determining enthalpy changes of chemical reactions e.g. Calorimetry is used to physically measure changes in enthalpy. in this a level and ib chemistry video, hazel describes how you carry out an. in. Calorimeter Experiment A Level.
From www.researchgate.net
The temperaturevstime graph for the of H 2 O 2 (aq Calorimeter Experiment A Level the essence of calorimetry in chemistry lies in its ability to link heat changes to molar quantities. the heat flows into the container of water held above the reaction. How much the temperature of the water changes is based on how much energy is. in the calorimetric experiment, substances are mixed in an insulated container, known as. Calorimeter Experiment A Level.
From atlas.cern
Progress on the level1 calorimeter trigger ATLAS Experiment at CERN Calorimeter Experiment A Level in the calorimetric experiment, substances are mixed in an insulated container, known as a calorimeter, and the temperature of the container is measured over a period of time. How much the temperature of the water changes is based on how much energy is. In an enthalpy change practical, the temperature change needs to be accurately measured. Calorimetry is used. Calorimeter Experiment A Level.
From grade12uchem.weebly.com
Calorimetry latest copy of grade 12 U Calorimeter Experiment A Level How much the temperature of the water changes is based on how much energy is. this page describes experimental methods for determining enthalpy changes of chemical reactions e.g. Using a simple calorimeter and a bomb. In an enthalpy change practical, the temperature change needs to be accurately measured. a level chemistry aqa 1.4.2. The temperature data is then. Calorimeter Experiment A Level.
From cider.uoregon.edu
Heat of Neutralization Demonstration and Simulation HCl + NaOH CIDER Calorimeter Experiment A Level Calorimetry is used to physically measure changes in enthalpy. How much the temperature of the water changes is based on how much energy is. in this a level and ib chemistry video, hazel describes how you carry out an. a level chemistry aqa 1.4.2. the heat flows into the container of water held above the reaction. The. Calorimeter Experiment A Level.
From exozspczc.blob.core.windows.net
Calorimeter Experiment Introduction at Jose Evans blog Calorimeter Experiment A Level Calorimetry is used to physically measure changes in enthalpy. a level chemistry aqa 1.4.2. the essence of calorimetry in chemistry lies in its ability to link heat changes to molar quantities. Using a simple calorimeter and a bomb. the heat flows into the container of water held above the reaction. How much the temperature of the water. Calorimeter Experiment A Level.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimeter Experiment A Level Calorimetry is used to physically measure changes in enthalpy. a level chemistry aqa 1.4.2. How much the temperature of the water changes is based on how much energy is. In an enthalpy change practical, the temperature change needs to be accurately measured. the essence of calorimetry in chemistry lies in its ability to link heat changes to molar. Calorimeter Experiment A Level.
From www.tessshebaylo.com
Equation For Calorimetry Specific Heat Tessshebaylo Calorimeter Experiment A Level Using a simple calorimeter and a bomb. The temperature data is then plotted, and extrapolation used to determine the temperature change on reaction of the substances. the heat flows into the container of water held above the reaction. In an enthalpy change practical, the temperature change needs to be accurately measured. in the calorimetric experiment, substances are mixed. Calorimeter Experiment A Level.
From www.researchgate.net
Block diagram of the Level1 Calorimeter Trigger, showing the main Calorimeter Experiment A Level How much the temperature of the water changes is based on how much energy is. In an enthalpy change practical, the temperature change needs to be accurately measured. The temperature data is then plotted, and extrapolation used to determine the temperature change on reaction of the substances. a level chemistry aqa 1.4.2. in this a level and ib. Calorimeter Experiment A Level.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals Calorimeter Experiment A Level the essence of calorimetry in chemistry lies in its ability to link heat changes to molar quantities. The temperature data is then plotted, and extrapolation used to determine the temperature change on reaction of the substances. in this a level and ib chemistry video, hazel describes how you carry out an. the heat flows into the container. Calorimeter Experiment A Level.
From www.savemyexams.co.uk
Biomass (5.3.3) AQA A Level Biology Revision Notes 2017 Save My Exams Calorimeter Experiment A Level a level chemistry aqa 1.4.2. How much the temperature of the water changes is based on how much energy is. Calorimetry is used to physically measure changes in enthalpy. in this a level and ib chemistry video, hazel describes how you carry out an. In an enthalpy change practical, the temperature change needs to be accurately measured. . Calorimeter Experiment A Level.
From www.markedbyteachers.com
The purpose of this experiment was to measure the specific heat Calorimeter Experiment A Level in the calorimetric experiment, substances are mixed in an insulated container, known as a calorimeter, and the temperature of the container is measured over a period of time. the heat flows into the container of water held above the reaction. a level chemistry aqa 1.4.2. Calorimetry is used to physically measure changes in enthalpy. How much the. Calorimeter Experiment A Level.
From galvinconanstuart.blogspot.com
Which Diagram Is A Bomb Calorimeter General Wiring Diagram Calorimeter Experiment A Level a level chemistry aqa 1.4.2. the essence of calorimetry in chemistry lies in its ability to link heat changes to molar quantities. in this a level and ib chemistry video, hazel describes how you carry out an. the heat flows into the container of water held above the reaction. How much the temperature of the water. Calorimeter Experiment A Level.
From ar.inspiredpencil.com
Heat Of Combustion Lab Calorimeter Experiment A Level How much the temperature of the water changes is based on how much energy is. a level chemistry aqa 1.4.2. Using a simple calorimeter and a bomb. the heat flows into the container of water held above the reaction. the essence of calorimetry in chemistry lies in its ability to link heat changes to molar quantities. . Calorimeter Experiment A Level.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download Calorimeter Experiment A Level in this a level and ib chemistry video, hazel describes how you carry out an. a level chemistry aqa 1.4.2. this page describes experimental methods for determining enthalpy changes of chemical reactions e.g. the heat flows into the container of water held above the reaction. Using a simple calorimeter and a bomb. the essence of. Calorimeter Experiment A Level.
From drivenheisenberg.blogspot.com
Which Diagram Is A Bomb Calorimeter Drivenheisenberg Calorimeter Experiment A Level Calorimetry is used to physically measure changes in enthalpy. in this a level and ib chemistry video, hazel describes how you carry out an. this page describes experimental methods for determining enthalpy changes of chemical reactions e.g. The temperature data is then plotted, and extrapolation used to determine the temperature change on reaction of the substances. How much. Calorimeter Experiment A Level.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimeter Experiment A Level Calorimetry is used to physically measure changes in enthalpy. the essence of calorimetry in chemistry lies in its ability to link heat changes to molar quantities. In an enthalpy change practical, the temperature change needs to be accurately measured. How much the temperature of the water changes is based on how much energy is. Using a simple calorimeter and. Calorimeter Experiment A Level.